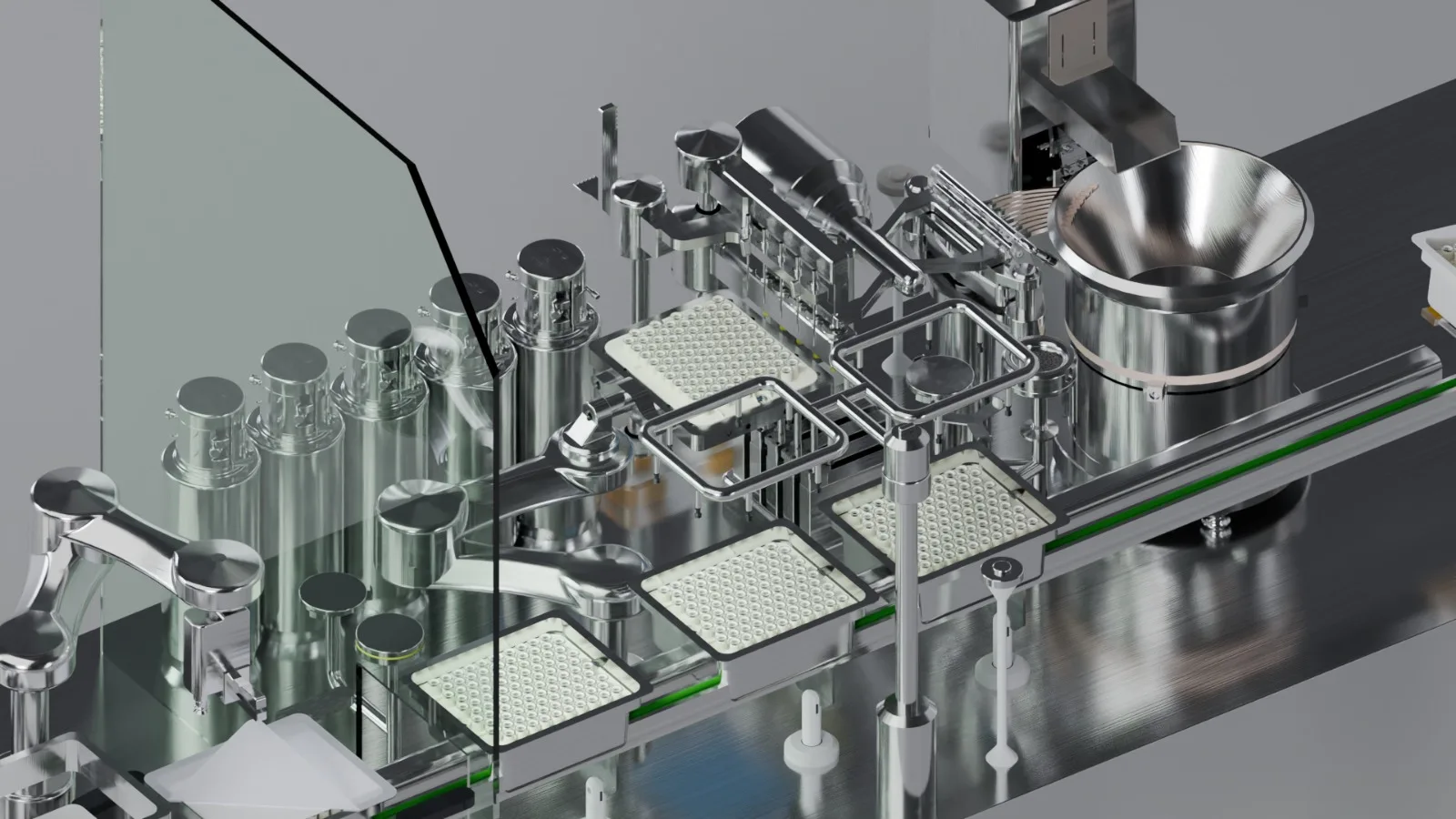
Nested Fill-Finish System
The Aseptic filling process from De-Bagging, De-Lidding, Filling and Vacuum Stoppering for RTU Nested Vials, Syringes and Cartridge containers.
Key Features and Benefits

- Programmable 100% fill weight IPC – consistent fill weight, weight IPC batch release with full confidence.
- Class A VHP (Vaporized Hydrogen Peroxide) Resistance Robot – ensures reliable, contamination-free operation in aseptic environments.
- Equipped with Electronic Batch Record(EBR) – Audit trial, User management.
- Vacuum Stoppering System – Minimize air entrapment for oxygen-sensitive product.
- Environment Monitoring – Real-time non-viable particle monitoring & Viable microbial continuous monitoring.
- Regulatory Compliance – Engineered to meet all relevant Annex 1, cGMP, GAMP 5 and 21CFR Part 11 requirements.
- Documentation – Full Validation document DQ (RA, FSDS, HDS, SDS, CSV) , IQ, OQ.
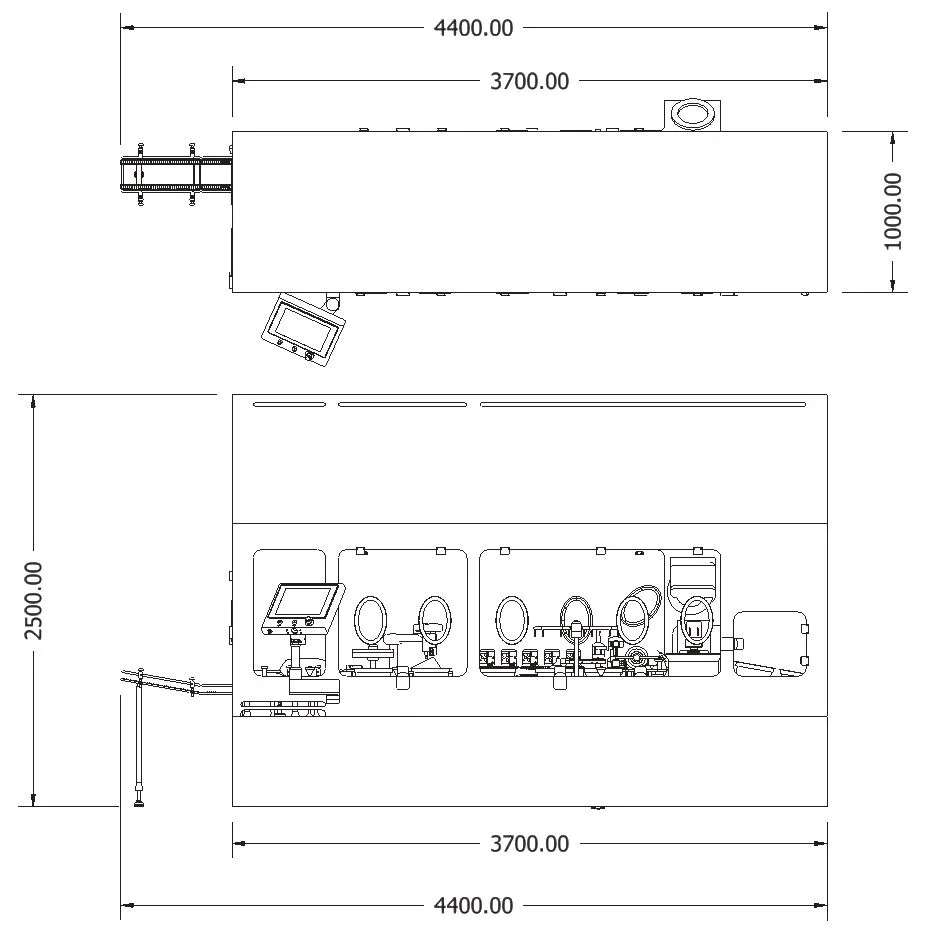
Machine Specification
| HFN60 | HFN120 | HFN180 | HFN350 | |
|---|---|---|---|---|
| Number of pumps | 1 | 2 | 5 | 10 |
| Speed PFS 1ml long (upm) | 30 | 70 | 150 | 300 |
| Speed Vial 2R (upm) | 25 | 65 | 130 | 300 |
| Speed Cartridge 3ml (upm) | 20 | 40 | 75 | 150 |
| Dosing Range | 0.1 – 20ml | |||
| Container | Nested RTU PFS, Vial, Cartridge | |||
| Plug | Rubber Stopper | |||
| Voltage | 380V – 415V, 50Hz | |||
| Air | 6 bar | |||
Frequently Asked Questions (FAQs)
How does the 100% Fill Weight IPC improve product quality and regulatory compliance?
The programmable 100% Fill Weight In-Process Control (IPC) ensures every vial, syringe, or cartridge is filled within the validated tolerance. This prevents underfills or overfills—common causes of batch rejections—and automatically records fill weights in real time, supporting data integrity, confident batch release, and compliance with Annex 1 and FDA 21 CFR Part 11.
Why is the Class A VHP-Resistant Robot important for aseptic production?
Sterility is critical in aseptic fill-finish. The Class A VHP-resistant robot handles nested containers in a contamination-free zone, ensuring safe, sterile operations even during long runs. Its VHP resistance ensures reliability and reduces downtime from manual decontamination.
How does the Vacuum Stoppering System reduce oxygen-related degradation?
The Vacuum Stoppering System minimizes air entrapment by applying controlled vacuum during stopper placement. This ensures a tight seal and protects product stability throughout its shelf life.
What are the benefits of having an integrated Electronic Batch Record (EBR) system?
Manual batch documentation risks errors and compliance issues. The NestFill HFN180’s EBR automates audit trails, user management, and traceable data recording, simplifying QA/QC review, speeding batch release, and ensuring compliance with GAMP 5 and data integrity standards.
How does the system ensure aseptic control during production?
NestFill continuously monitors the environment with non-viable particle counting and microbial checks. Immediate alerts for deviations allow corrective action before contamination occurs, addressing a major challenge in aseptic processing.