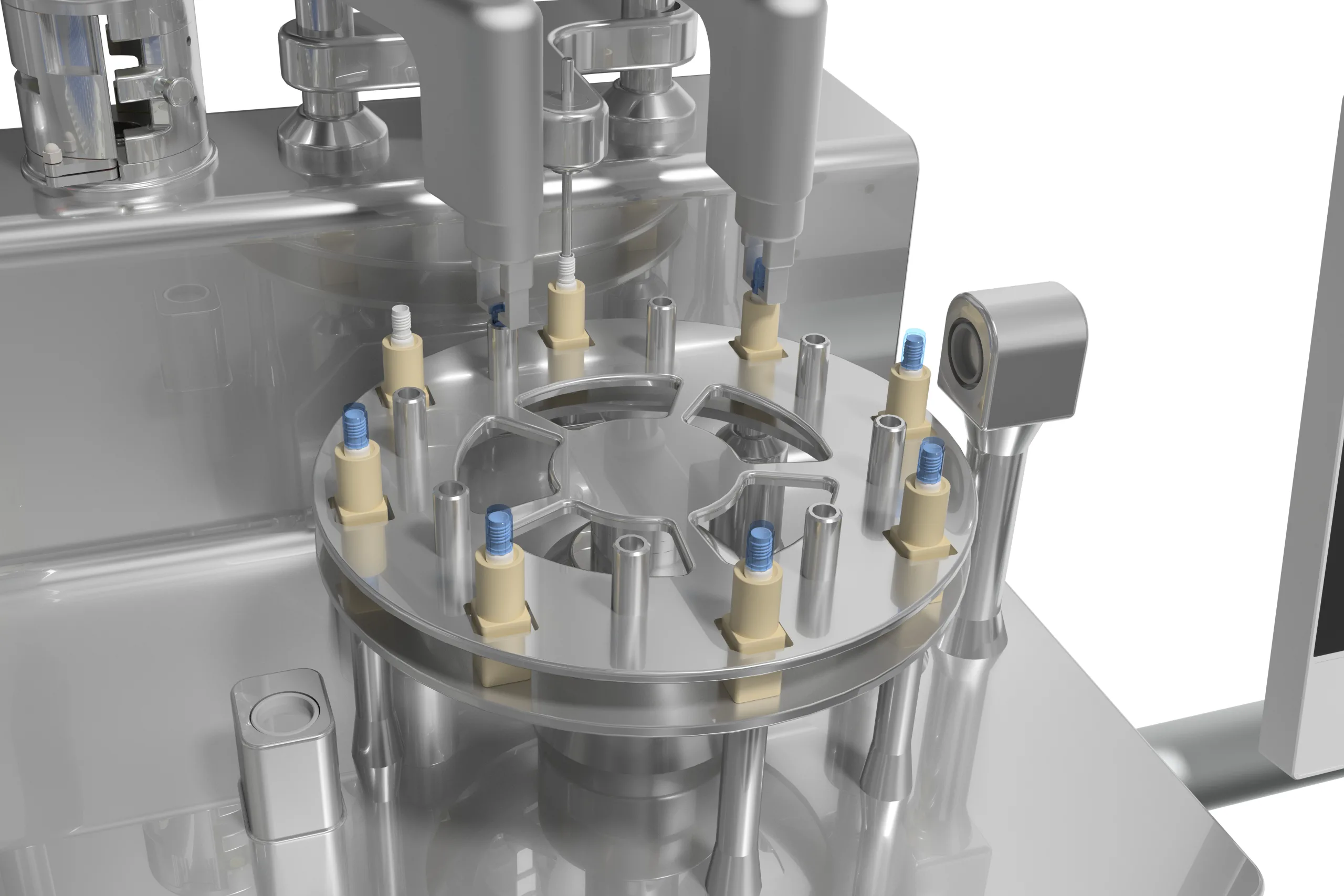
Cryovial Fill-Finish System
A New standard to fill temperature sensitive product like Cell and Gene Therapy by automatic uncapping, Filling, Recapping onto a Cryovial in rack , immediate transfer into controlled rate freezer without additional vial handing.
Key Features and Benefits

- Installation Flexibility – Stand alone design, can be fit into any brand of Bio Safety Cabinet or Isolator.
- 100% Fill weight IPC – 100% tare in / tare out, zero reject for inconsistent fill weight, batch release with full confidence.
- Sterility – Minimum vial open time, each vial will be capped immediately after filling to minimize risk of contamination align with Annex 1 Contamination Control Strategy (CCS).
- 100% Capping inspection IPC – No Sampling, 100% automatic vision inspection on capping quality like slanted cap, missing cap, avoid inconsistent human check.
- Barcode Scanning – Come with standard feature for barcode vial for better traceability.
- FDA 21CFRPart 11 – Compliant with Electronic Batch Record(EBR), Audit trial, User management.
- Documentation – Full Validation document DQ(RA, FSDS, HDS, SDS, CSV) , IQ, OQ.
Machine Specification
| Model | HCF20 |
|---|---|
| Machine | Filling and Capping |
| Speed | 720 uph |
| Dosing Range | 0.5ml – 5ml |
| Container | Cyrovial 1ml – 5ml |
| * with change part | |
| Closure | Screw on cap |
| Voltage | 240V single phase |
| Air | Not applicable |
| Installation | Isolator or Bio Safety Cabinet |
Frequently Asked Questions (FAQs)
How does the CryoFill system ensure accurate fill volumes?
CryoFill uses 100% IPC fill weight verification with tare-in/tare-out weighing for every cryovial. This eliminates sampling errors, prevents rejects, and ensures full batch release confidence—critical for high-value cell and gene therapy products.
How does the CryoFill design minimize contamination risk during aseptic filling?
CryoFill minimizes open-vial exposure by automatically uncapping, filling, and re-capping each cryovial within a controlled isolator or biosafety cabinet. This rapid process aligns with EU GMP Annex 1 CCS, reducing microbial and particle contamination risks for aseptic ATMP manufacturing.
How does the CryoFill system verify capping integrity to meet regulatory requirements?
CryoFill performs 100% automatic vision inspection for capping torque and closure integrity, detecting missing, slanted, or loose caps. Real-time IPC monitoring on every vial ensures traceable records compliant with FDA 21 CFR Part 11 and data integrity standards.
Can the CryoFill integrate seamlessly into existing cleanroom or isolator setups?
Yes. CryoFill’s compact, stand-alone design allows flexible installation into any isolator or biosafety cabinet, ideal for small-batch, high-value biologics where space and environmental control are critical.
How does CryoFill enhance traceability and documentation for regulatory audits?
CryoFill includes barcode scanning for vial identification and full EBR capability. All operations are recorded with audit trails and user management under FDA 21 CFR Part 11. Comprehensive validation documents (DQ, IQ, OQ, FSDS, CSV) simplify GMP audits and qualification.