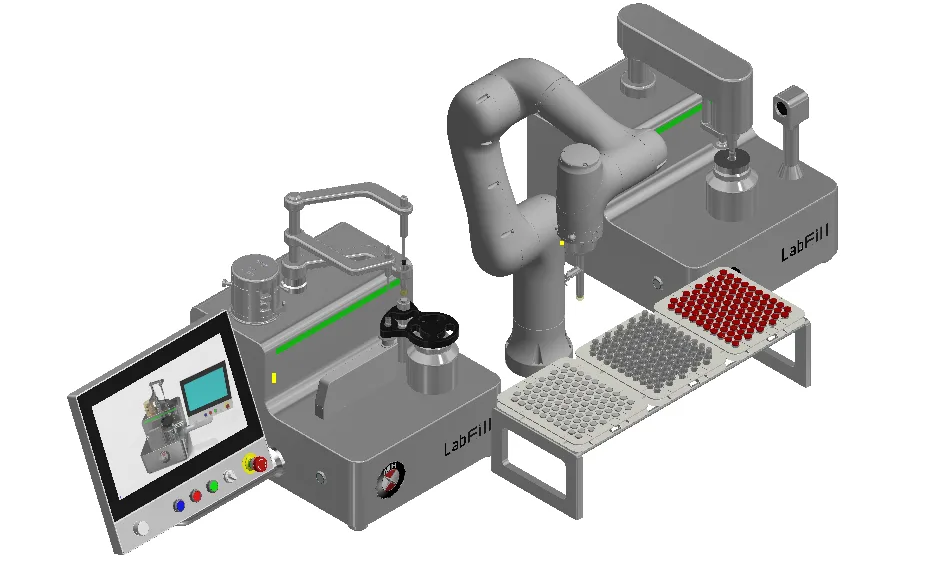
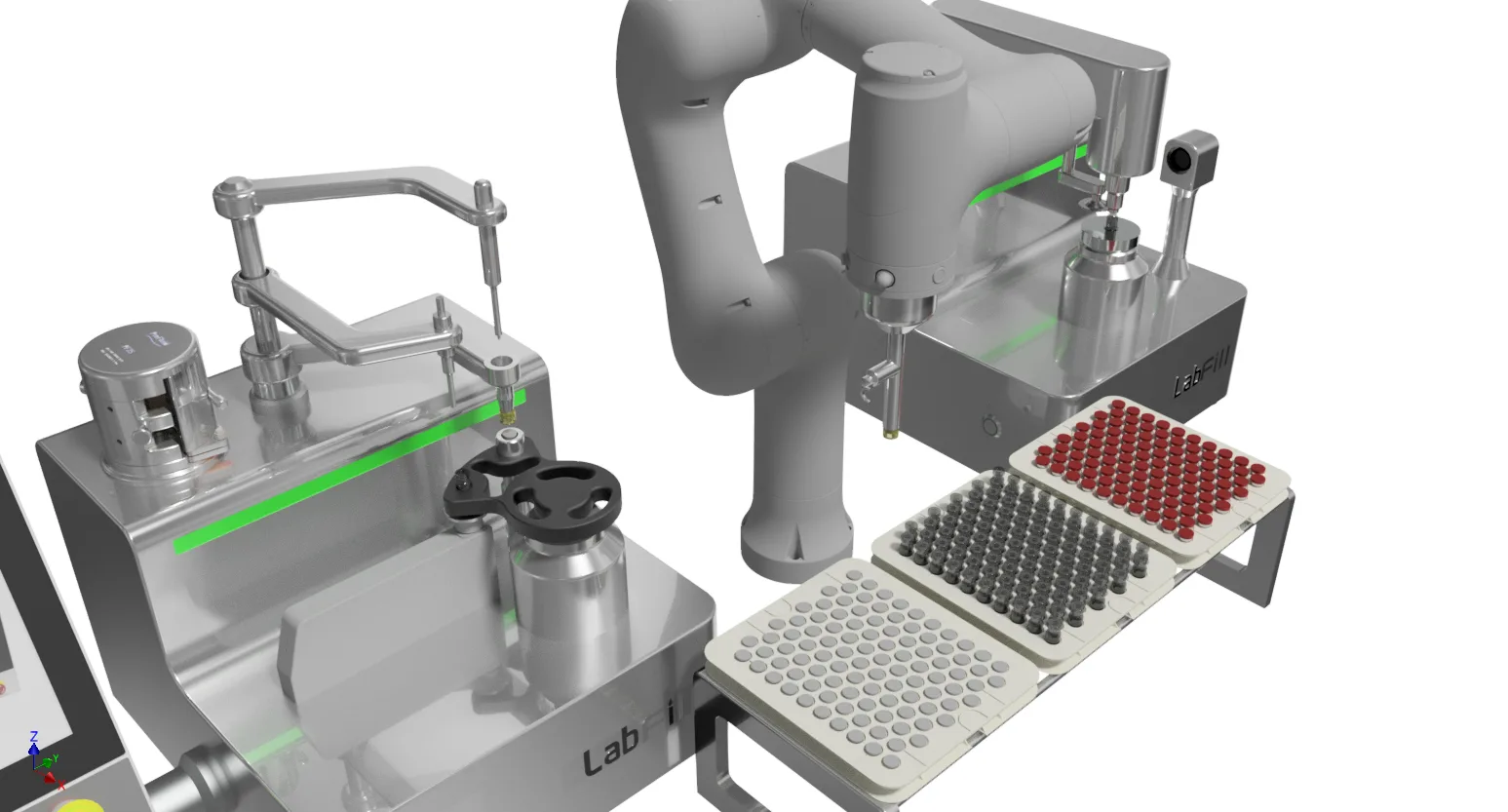
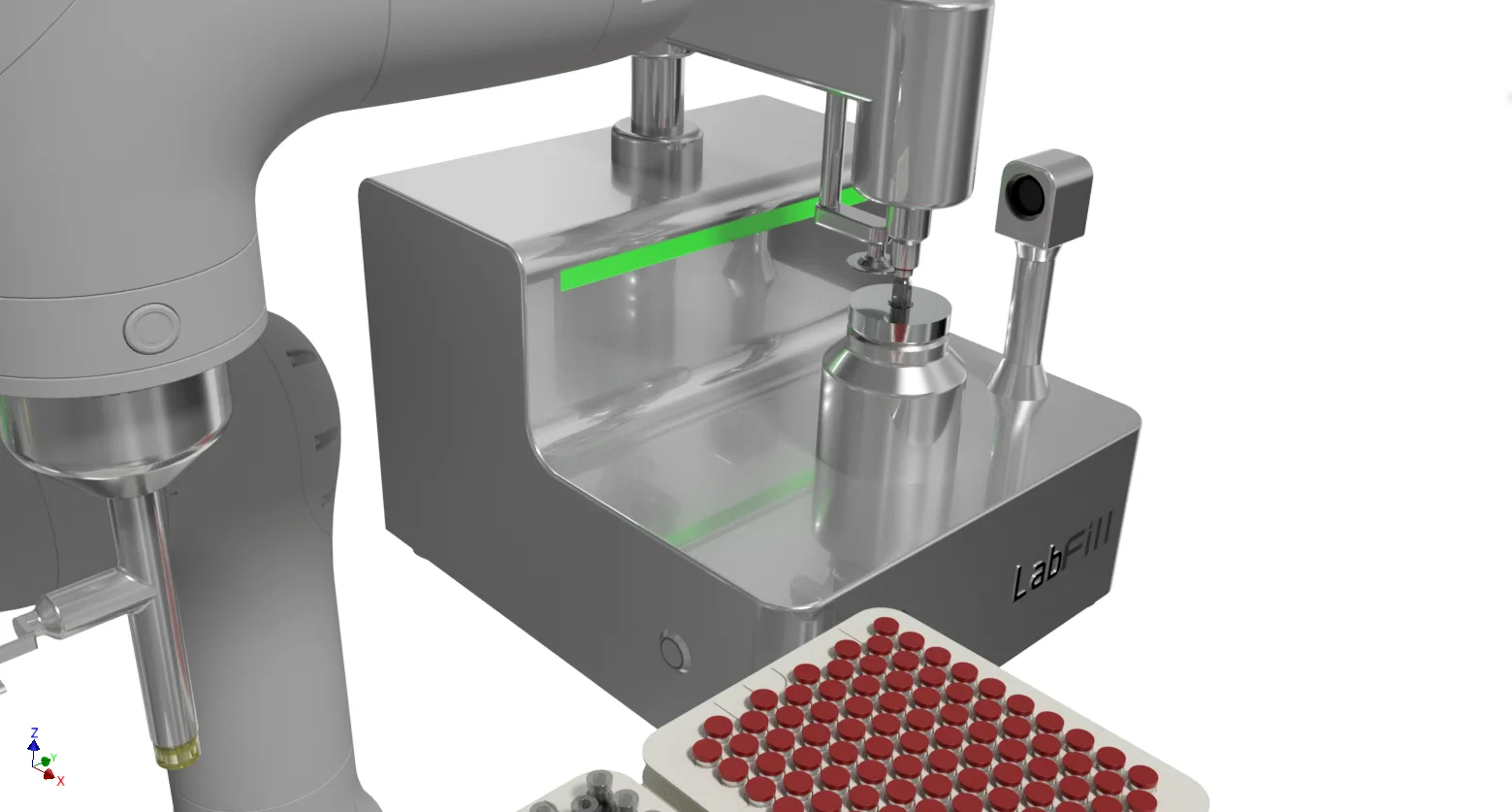
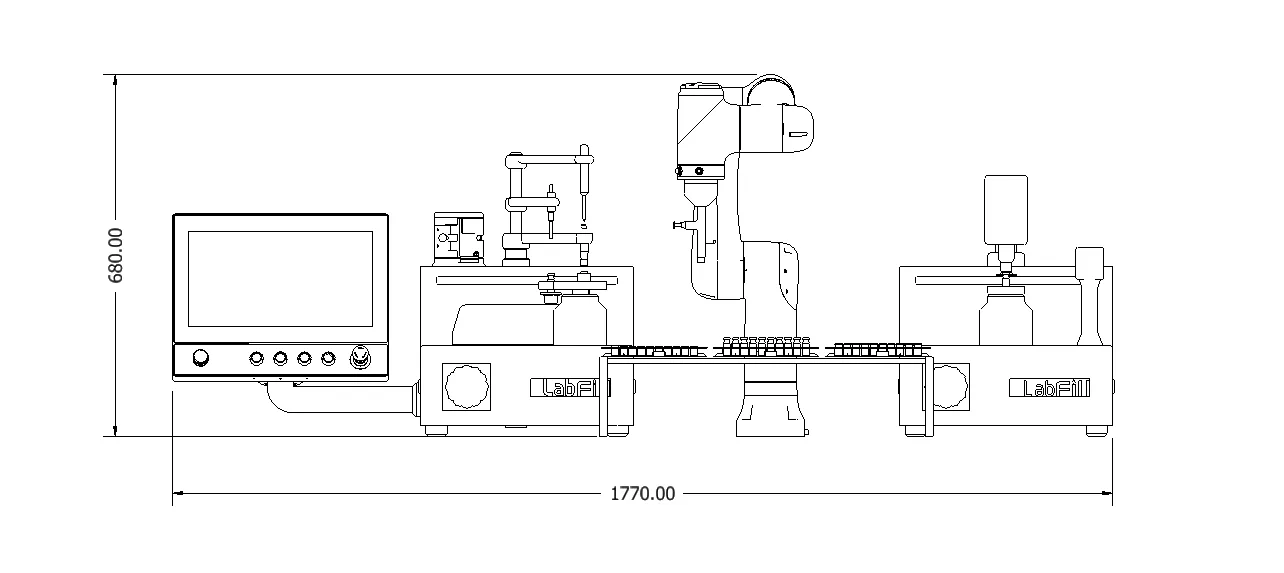
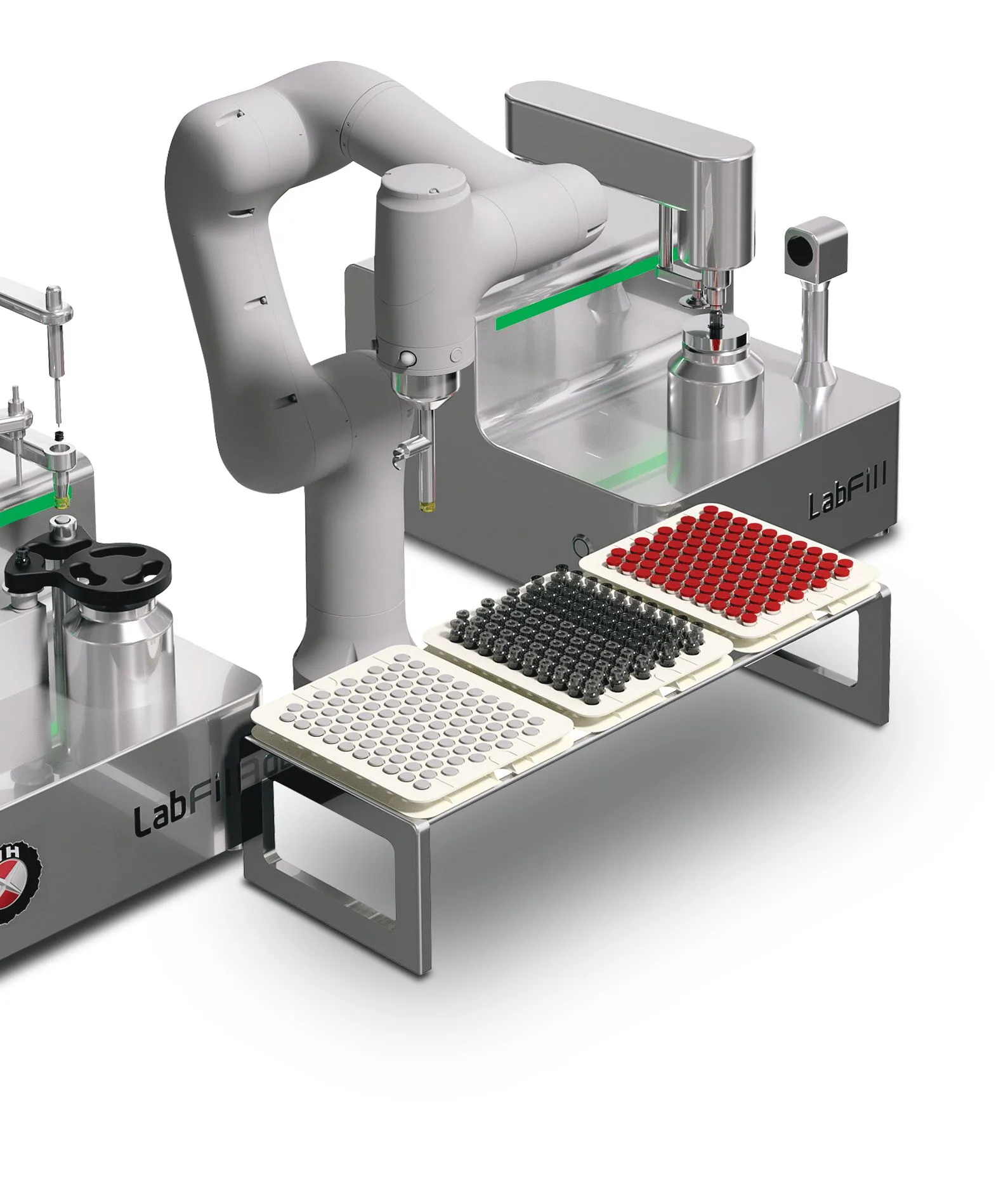
Lab Fill-Finish System
A New level of Lab scale Filling, Stoppering and Vial Sealing used on batch micro manufacturing, clinical lab and hospital for Biologics, Cell & Gene Therapies, ATMP and Vaccine Filling application.
Key Features and Benefits

- Process Flexibility – One machine for Filling and Stoppering, solution for Capex saving, space saving.
- Container Flexibility – One machine handle Vial, PFS, Cartridge, ideal for high mix, low volume micro batch.
- 100% Fill weight IPC – 100% tare in / tare out, zero reject for inconsistent fill weight, batch release with full confidence.
- 100% Compression force IPC – 100% ensure securely seal maintaining product sterility, align with Annex 1 for container closure integrity(CCI).
- 100% Sealing inspection IPC – No Sampling, 100% automatic vision inspection, avoid inconsistent human check.
- FDA21CFRPart 11 – Compliant with Electronic Batch Record(EBR), Audit trial, User management.
- Documentation – Full Validation document DQ(RA, FSDS, HDS, SDS, CSV) , IQ, OQ.
Optional
- Robotic handling – no human intervention, no contamination.
Machine Specification
| Model | HLF10 | HLC10 |
|---|---|---|
| Machine | Filling and Stoppering | Cap Sealing |
| Speed | 360uph | 360uph |
| Dosing Range | 0.5ml – 20ml | – |
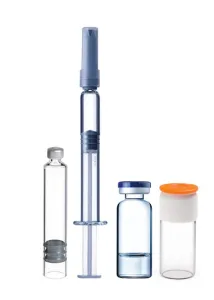
| Container | Vial 1ml – 20ml | |
| PFS 1ml – 20ml Vial 1ml – 20ml | Vial 1ml – 20ml | |
| Cartridge 1ml – 3ml | ||
| * with change part | ||
| Closure | Vial Stoppering | Aluminium flip off cap |
| RayDyLyo® Push-Fit cap | RayDyLyo® Push-Fit cap | |
| Cartridge Vent Tube Stoppering | ||
| Voltage | 240V single phase | 240V single phase |
| Air | 6 bar | – |
| Installation | Isolator or Bio Safety Cabinet | Isolator or Bio Safety Cabinet |
Frequently Asked Questions (FAQs)
How does LabFill ensure accurate and consistent fill volumes?
LabFill integrates 100% Fill Weight IPC with tare-in/tare-out precision weighing. Every vial, cartridge, or prefilled syringe is verified for exact fill, preventing rejects. This is critical for expensive biologics and cell & gene therapy materials, where product loss and variability impact yield and compliance.
Why is 100% Cap Compression Force monitoring important for sterility?
LabFill measures sealing pressure for every container in real time, ensuring secure, validated closures. This maintains Container Closure Integrity (CCI) per EU Annex 1, preventing microleaks that could compromise sterility or product stability.
How does LabFill reduce human error and contamination risk?
Optional robotic handling enables fully automated, hands-free filling, stoppering, and sealing. Minimizing operator intervention reduces contamination risks, improves reproducibility, and supports GMP aseptic assurance.
Can LabFill handle different container types?
Yes. LabFill processes vials, prefilled syringes, and cartridges using quick-change parts, ideal for high-mix, low-volume micro-batches, eliminating the need for multiple machines.
How does LabFill support regulatory compliance and electronic documentation?
The system complies with FDA 21 CFR Part 11, with Electronic Batch Records, audit trails, and user management. Documentation (DQ, IQ, OQ, CSV) ensures seamless validation, traceability, and GMP compliance.